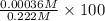Chemistry, 29.08.2019 18:00 lhmsokol56
Calculate the percent ionization of nitrous acid in a solution that is 0.222 m in nitrous acid (hno3) and 0.278 m in potassium nitrite (kno2). the acid dissociation constant of nitrous acid is 4.50 x 10-4 a) 55.6 b) 0.162 c) 15.5 d) 2.78 * 10-3

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Calculate the percent ionization of nitrous acid in a solution that is 0.222 m in nitrous acid (hno3...
Questions

World Languages, 26.11.2021 14:00

English, 26.11.2021 14:00

Social Studies, 26.11.2021 14:00

Computers and Technology, 26.11.2021 14:00





History, 26.11.2021 14:00

Chemistry, 26.11.2021 14:00


Advanced Placement (AP), 26.11.2021 14:00

SAT, 26.11.2021 14:00

English, 26.11.2021 14:00

Health, 26.11.2021 14:00

English, 26.11.2021 14:00

History, 26.11.2021 14:00



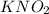 is 0.278 M and it is completely ionized into
is 0.278 M and it is completely ionized into 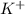 and
and 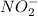 .
.![[KNO_{2}]](/tpl/images/0209/3896/fb191.png) =
= ![[NO_{2}]](/tpl/images/0209/3896/53e25.png) = 0.278 M
= 0.278 M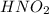 is 0.222 M.
is 0.222 M.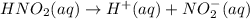
![K_{a} = \frac{[H^{+}][NO^{-}_{2}]}{[HNO_{2}]}](/tpl/images/0209/3896/218b7.png)

![[H^{+}]](/tpl/images/0209/3896/85507.png) is 0.00036 M. Therefore, percentage ionization of
is 0.00036 M. Therefore, percentage ionization of ![\frac{[H^{+}]}{[HNO_{2}]} \times 100](/tpl/images/0209/3896/26d17.png)