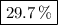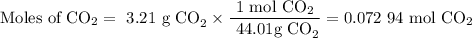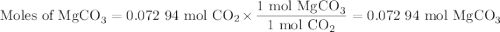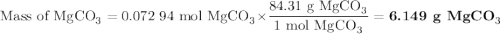Chemistry, 26.08.2019 23:30 chloiesierra29
A20.69 g sample of impure magnesium car- bonate was heated to complete decomposition according to the equation mgco3(s) → mgo(s) + co2(g) . after the reaction was complete, the solid residue (consisting of mgo and the original impurities) had a mass of 17.48 g. assum- ing that only the magnesium carbonate had decomposed, what was the percent of magne- sium carbonate in the original sample? answer in units of %.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
An occluded front moves over the farmland that has been experiencing drought conditions. what change in weather will this front likely bring? a. gray skies, but no rain b. an extended period of rain c. more dry air and sunny skies d. violent, short-lived thunderstorms
Answers: 3

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2
You know the right answer?
A20.69 g sample of impure magnesium car- bonate was heated to complete decomposition according to th...
Questions



Mathematics, 11.01.2021 19:30

Mathematics, 11.01.2021 19:30


Chemistry, 11.01.2021 19:30



Chemistry, 11.01.2021 19:30

Mathematics, 11.01.2021 19:30



Mathematics, 11.01.2021 19:30


English, 11.01.2021 19:30

Mathematics, 11.01.2021 19:30

Mathematics, 11.01.2021 19:30

Mathematics, 11.01.2021 19:30