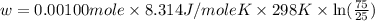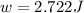Chemistry, 23.08.2019 05:30 coopyishome
Apiston chamber filled with ideal gas is kept in a constant-temperature bath at 25.0°c. the piston expands from 25.0 ml to 75.0 ml very, very slowly, as illustrated in the figure below. if there is 0.00100 mole of ideal gas in the chamber, calculate the work done by the system

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Apiston chamber filled with ideal gas is kept in a constant-temperature bath at 25.0°c. the piston e...
Questions

Mathematics, 09.01.2020 03:31



Mathematics, 09.01.2020 03:31

Biology, 09.01.2020 03:31


Biology, 09.01.2020 03:31


Biology, 09.01.2020 03:31

Biology, 09.01.2020 03:31


Biology, 09.01.2020 03:31


Mathematics, 09.01.2020 03:31


Biology, 09.01.2020 03:31

Biology, 09.01.2020 03:31



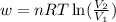
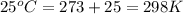
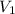 = initial volume of gas = 25 mL
= initial volume of gas = 25 mL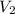 = final volume of gas = 75 mL
= final volume of gas = 75 mL