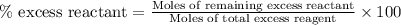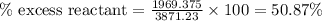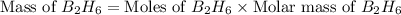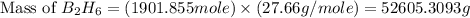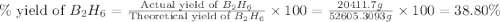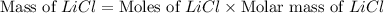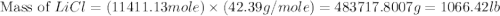Diborane, b2h6 a possible rocket propellant, can be made by using lithium hydride (lih): 6 lih+ 2 bcl2àb2h6+ 6 licl . if you mix 200 lb of lih with 1000 lb of bcl3 , you recover 45 lb of b2h6. determine (a) limiting reactant (b) the excess reactant (c) the percent excess reactant (d) the percent conversion of lih to b2h6 (e) lb of licl produced

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

You know the right answer?
Diborane, b2h6 a possible rocket propellant, can be made by using lithium hydride (lih): 6 lih+ 2 b...
Questions

Mathematics, 14.10.2021 01:00





Mathematics, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00




Mathematics, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00

Physics, 14.10.2021 01:00



Mathematics, 14.10.2021 01:00


Mathematics, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00

Mathematics, 14.10.2021 01:00

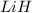
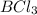
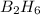 or percent conversion of
or percent conversion of 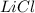 produced is, 1066.42 lb
produced is, 1066.42 lb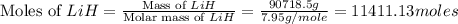
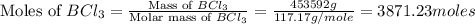
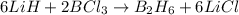
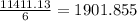 moles of
moles of 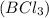 .
.