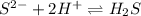Chemistry, 21.08.2019 05:30 emmareese2022
The best explanation for the dissolution of zns in dilute hcl is that a. the sulfide ion concentration is decreased by the formation of h2s. b. the zinc ion is amphoteric. c. the zinc ion concentration is decreased by the formation of a chloro complex. d. the sulfide ion concentration is decreased by oxidation to sulfur. e. the solubility product of zncl2 is less than that of zns.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
The best explanation for the dissolution of zns in dilute hcl is that a. the sulfide ion concentrati...
Questions


Mathematics, 29.11.2021 05:30


Mathematics, 29.11.2021 05:30




Mathematics, 29.11.2021 05:30

Mathematics, 29.11.2021 05:30

Mathematics, 29.11.2021 05:30



Mathematics, 29.11.2021 05:30

Mathematics, 29.11.2021 05:30


Mathematics, 29.11.2021 05:30


Physics, 29.11.2021 05:30


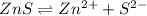
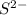 is a strong conjugate base of weak acid
is a strong conjugate base of weak acid 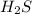 .
.