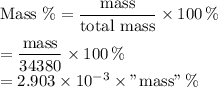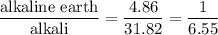The seven most abundant ions in seawater make up more than 99% by mass of the dissolved compounds. here are their abundances in units of mg ion/kg seawater: chloride 18,980; sodium 10,560; sulfate 2650; magnesium 1270; calcium 400; potassium 380; hydrogen carbonate 140. (a) what is the mass 96 of each ion in seawater? (b) what percent of the total mass of ions is sodium ion? (c) how does the total mass % of alkaline earth metal ions compare with the total mass 96 of alkali metal ions? (d) which make up the larger mass fraction of dissolved components, anions or cations?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

You know the right answer?
The seven most abundant ions in seawater make up more than 99% by mass of the dissolved compounds. h...
Questions

Mathematics, 06.05.2020 20:25

















Mathematics, 06.05.2020 20:25