
Suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(ii) chloride, which would react with silver nitrate solution like this: fecl2 (aq) + 2agno3 (aq) → 2agcl (s) + feno32 (aq) the chemist adds 14.0m m silver nitrate solution to the sample until silver chloride stops forming. she then washes, dries, and weighs the precipitate. she finds she has collected 6.9mg of silver chloride. calculate the concentration of iron(ii) chloride contaminant in the original groundwater sample. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Suppose an epa chemist tests a 200.ml sample of groundwater known to be contaminated with iron(ii) c...
Questions



Mathematics, 09.11.2020 18:40


English, 09.11.2020 18:40

Health, 09.11.2020 18:40

Mathematics, 09.11.2020 18:40

History, 09.11.2020 18:40

Mathematics, 09.11.2020 18:40


Computers and Technology, 09.11.2020 18:40

Spanish, 09.11.2020 18:40



Mathematics, 09.11.2020 18:40

History, 09.11.2020 18:40

Mathematics, 09.11.2020 18:40


Social Studies, 09.11.2020 18:40

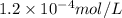 .
.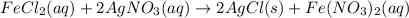
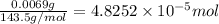
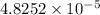 moles of silver chloride will be obtained from:
moles of silver chloride will be obtained from: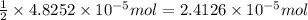
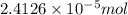
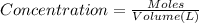
![[FeCl_2]=\frac{2.4126\times 10^{-5} mol}{0.2 L}=0.00012063 mol/L](/tpl/images/0173/9764/69c0f.png)
![[FeCl_2]=1.2063\times 10^{-4} mol/L\approx 1.2\times 10^{-4} mol/L](/tpl/images/0173/9764/77e47.png)



