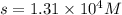Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

You know the right answer?
Silver chromate, ag. cro, has a kp of 9.00x 1012 calculate the solubility in mol/l of silver chromat...
Questions

Mathematics, 03.02.2020 16:46



History, 03.02.2020 16:46


Mathematics, 03.02.2020 16:46



Biology, 03.02.2020 16:46


Chemistry, 03.02.2020 16:46


Geography, 03.02.2020 16:46


Mathematics, 03.02.2020 16:46



Chemistry, 03.02.2020 16:46


History, 03.02.2020 16:46

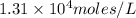
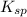
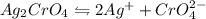
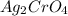 = S mol/L
= S mol/L
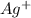 and 1 mole of
and 1 mole of 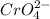 .
.
![K_{sp}=[Ag^+]^2[CrO_4^{2-}]](/tpl/images/0173/1541/cfca0.png)
![9.00\times 10^{12}=[2s]^2[s]=4s^3](/tpl/images/0173/1541/7b180.png)