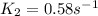Chemistry, 08.08.2019 05:30 sparky1234
Areaction is found to have an activation energy of 108 kj/mol. if the rate constant for this reaction is 4.60 x 10-6 s-1 at 275 k, what is the rate constant at 366 k? 0.58 1/s

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
Areaction is found to have an activation energy of 108 kj/mol. if the rate constant for this reactio...
Questions



Biology, 19.06.2020 07:57


Mathematics, 19.06.2020 07:57




Chemistry, 19.06.2020 07:57

Mathematics, 19.06.2020 07:57

Mathematics, 19.06.2020 07:57

Mathematics, 19.06.2020 07:57

Biology, 19.06.2020 07:57



English, 19.06.2020 07:57

History, 19.06.2020 07:57

Mathematics, 19.06.2020 07:57


History, 19.06.2020 07:57

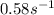
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0173/1540/6d953.png)
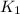 =rate constant at
=rate constant at 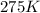 =
= 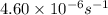
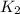 = rate constant at
= rate constant at 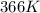 = ?
= ?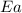 = activation energy for the reaction = 108kJ/mol=108000 J/mol
= activation energy for the reaction = 108kJ/mol=108000 J/mol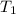 = initial temperature =
= initial temperature = 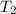 = final temperature =
= final temperature = ![\log (\frac{K_2}{4.60\times 10^{-6}})=\frac{108000}{2.303\times 8.314J/mole.K}[\frac{1}{275K}-\frac{1}{366K}]](/tpl/images/0173/1540/60a14.png)