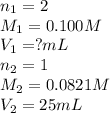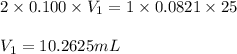Chemistry, 06.08.2019 01:30 heavendavis101
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(aq) + 2 h2o(l) calculate the volume of 0.100 m sulfuric acid required to neutralize 25.0 ml of 0.0821 m koh.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
Sulfuric acid (h2so4) reacts with potassium hydroxide (koh) as follows. h2so4(aq) + 2 koh(aq) k2so4(...
Questions



Computers and Technology, 02.06.2020 05:58


Mathematics, 02.06.2020 05:58

Mathematics, 02.06.2020 05:58

History, 02.06.2020 05:58

Mathematics, 02.06.2020 05:58

Mathematics, 02.06.2020 05:58


Health, 02.06.2020 05:58




History, 02.06.2020 05:58

English, 02.06.2020 05:58

Biology, 02.06.2020 05:58


Arts, 02.06.2020 05:58

History, 02.06.2020 05:58

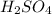 comes out to be 10.2625 mL.
comes out to be 10.2625 mL.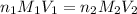
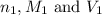 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 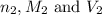 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.