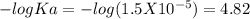Chemistry, 06.08.2019 01:30 darius12318
Suppose 10.0 ml of 2.00 mnaoh is added to (a) 0.780 l of pure water and (b) 0.780 l of a buffer solution that is 0.682 min butanoic acid (hc4h7o2) and 0.674 min butanoate ion (c4h7o2–). calculate the ph of (a) and (b) before and after the addition of the naoh. assume volumes are additive. (ka, hc4h7o2= 1.5 × 10-5)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Suppose 10.0 ml of 2.00 mnaoh is added to (a) 0.780 l of pure water and (b) 0.780 l of a buffer solu...
Questions

Mathematics, 10.11.2020 05:20

Mathematics, 10.11.2020 05:20


Mathematics, 10.11.2020 05:20



Geography, 10.11.2020 05:20

Engineering, 10.11.2020 05:20


Mathematics, 10.11.2020 05:20




Mathematics, 10.11.2020 05:20

Social Studies, 10.11.2020 05:20


Chemistry, 10.11.2020 05:20

Mathematics, 10.11.2020 05:20

Mathematics, 10.11.2020 05:20

Social Studies, 10.11.2020 05:20

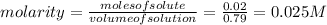
![pH=pKa+log\frac{[salt]}{[acid]}](/tpl/images/0171/9988/ec35f.png)