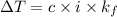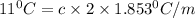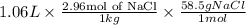Chemistry, 05.08.2019 22:30 westes0376
What mass of salt (nacl) should you add to 1.06 l of water in an ice cream maker to make a solution that freezes at -11.0 ∘c ? assume complete dissociation of the nacl and density of 1.00 g/ml for water.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3
You know the right answer?
What mass of salt (nacl) should you add to 1.06 l of water in an ice cream maker to make a solution...
Questions

English, 03.04.2021 07:10

Mathematics, 03.04.2021 07:10

Arts, 03.04.2021 07:10



Social Studies, 03.04.2021 07:10

Biology, 03.04.2021 07:10


English, 03.04.2021 07:10

Mathematics, 03.04.2021 07:20



Mathematics, 03.04.2021 07:20

Mathematics, 03.04.2021 07:20

Mathematics, 03.04.2021 07:20





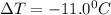
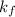 for water = 1.853 ^{0}C m, density = 1.00 g/mL
for water = 1.853 ^{0}C m, density = 1.00 g/mL