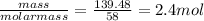Chemistry, 05.08.2019 22:30 izzynikkie
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in nail polish remover: c3h6o(l)+4 o2(g)→3 co2(g)+3 h2o(g)δh°rxn=−1790 kj if a bottle of nail polish remover contains 177 ml of acetone, how much heat is released by its complete combustion? the density of acetone is 0.788 g/ml.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2


Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
Consider the thermochemical equation for the combustion of acetone (c3h6o), the main ingredient in n...
Questions


Mathematics, 16.12.2020 19:30


Mathematics, 16.12.2020 19:30




Chemistry, 16.12.2020 19:30

Mathematics, 16.12.2020 19:30

Chemistry, 16.12.2020 19:30



English, 16.12.2020 19:30




English, 16.12.2020 19:30


Arts, 16.12.2020 19:30

Arts, 16.12.2020 19:30