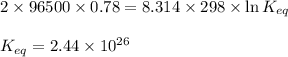In the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell arecu2+(aq)+2e−→cu(s) and fe(s)→fe2+(aq)+2e−

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this re...
Questions

Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Computers and Technology, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30



Mathematics, 20.05.2021 16:30

History, 20.05.2021 16:30


Mathematics, 20.05.2021 16:30


Mathematics, 20.05.2021 16:30

Physics, 20.05.2021 16:30

Mathematics, 20.05.2021 16:30

History, 20.05.2021 16:30


Mathematics, 20.05.2021 16:30

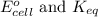 of the reaction is 0.78 V and
of the reaction is 0.78 V and 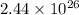 respectively.
respectively.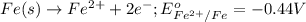
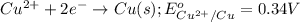

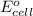 of the reaction, we use the equation:
of the reaction, we use the equation: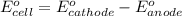
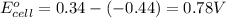

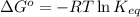
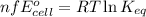
![25^oC=[273+25]=298K](/tpl/images/0171/8602/6a9f9.png)
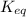 = equilibrium constant of the reaction = ?
= equilibrium constant of the reaction = ?