
Chemistry, 04.08.2019 05:10 cia196785920
15 points
a 0.100 m solution of cyanic acid (hcno) is 5.9% ionized. using this information, calculate
[cno], [h3o+], [hcno] and k, for cyanic acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
15 points
a 0.100 m solution of cyanic acid (hcno) is 5.9% ionized. using this information, ca...
a 0.100 m solution of cyanic acid (hcno) is 5.9% ionized. using this information, ca...
Questions

Mathematics, 03.11.2020 19:10


English, 03.11.2020 19:10


History, 03.11.2020 19:10


Mathematics, 03.11.2020 19:10

History, 03.11.2020 19:10

Mathematics, 03.11.2020 19:10

English, 03.11.2020 19:10


English, 03.11.2020 19:10


Mathematics, 03.11.2020 19:10

Mathematics, 03.11.2020 19:10

Mathematics, 03.11.2020 19:10



Mathematics, 03.11.2020 19:10

Health, 03.11.2020 19:10

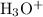 is 0.0059 mol/L,
is 0.0059 mol/L, 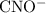 is 0.0059 mol/L, and HCHO is 0.094 mol/L. The value of k for cyanic acid has been 0.00037.
is 0.0059 mol/L, and HCHO is 0.094 mol/L. The value of k for cyanic acid has been 0.00037.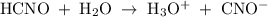
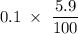
![\rm k\;=\;\dfrac{[H_3O^+]\;[CNO^-]}{[HCHO]}](/tpl/images/0168/2736/50271.png)
![\rm k\;=\;\dfrac{[0.0059]\;[0.0059]}{[0.094]}](/tpl/images/0168/2736/047ae.png)
![K = \dfrac{[\text{H$_{3}$O$^{+}$}][\text{CNO$^{-}$}] }{[\text{HCNO}]} = \dfrac{0.0059^{2}}{0.094} = \mathbf{3.7 \times 10^{-4}}](/tpl/images/0168/2736/ead7b.png)


