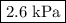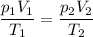Chemistry, 04.08.2019 05:10 kprincess16r
Asample of nitrogen is initially at a pressure of 1.7 kpa, a temperature of -10 c and a volume of 7.5 m3. then the volume is decreased to 3.8 m3. the temperature is decreased to 200 k. what is the final pressure of the nitrogen gas?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
Asample of nitrogen is initially at a pressure of 1.7 kpa, a temperature of -10 c and a volume of 7....
Questions



Mathematics, 31.08.2020 05:01

Spanish, 31.08.2020 05:01

Mathematics, 31.08.2020 05:01


Mathematics, 31.08.2020 05:01

History, 31.08.2020 05:01

Mathematics, 31.08.2020 05:01


Business, 31.08.2020 05:01

Spanish, 31.08.2020 05:01




Mathematics, 31.08.2020 05:01


Mathematics, 31.08.2020 05:01


Geography, 31.08.2020 05:01