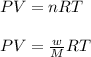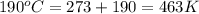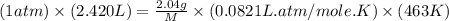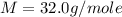Chemistry, 31.07.2019 21:20 rashawng2005
Asample of an unknown compound is vaporized at 190.°c. the gas produced has a volume of 2420.ml at a pressure of 1.00 atm, and it weighs 2.04 g. assuming the gas behaves as an ideal gas under these conditions, calculate the molar mass of the compound. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
Asample of an unknown compound is vaporized at 190.°c. the gas produced has a volume of 2420.ml at a...
Questions


Mathematics, 04.02.2021 22:20

Biology, 04.02.2021 22:20

Mathematics, 04.02.2021 22:20

Mathematics, 04.02.2021 22:20

Mathematics, 04.02.2021 22:20

Chemistry, 04.02.2021 22:20


Social Studies, 04.02.2021 22:20


Mathematics, 04.02.2021 22:20

History, 04.02.2021 22:20



History, 04.02.2021 22:20

Mathematics, 04.02.2021 22:20

Mathematics, 04.02.2021 22:20


Mathematics, 04.02.2021 22:20