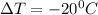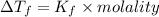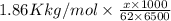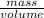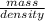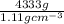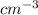Chemistry, 31.07.2019 20:30 shelbylynn17
Acommon antifreeze for car radiators is ethylene glycol, ch2(oh )ch2(oh ). how many millilite~s of this substance would you add to 6.5 l of water 1n the radiator if the coldest day in winter is - 20°c? would you keep this substance in the radiator in the summer to prevent the water from boiling? (the density and boiling point of ethylene glycol are 1.11 g cm^-3 and 470 k repsectively.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Acommon antifreeze for car radiators is ethylene glycol, ch2(oh )ch2(oh ). how many millilite~s of t...
Questions

Mathematics, 18.11.2020 02:20


Chemistry, 18.11.2020 02:20

Chemistry, 18.11.2020 02:20



Mathematics, 18.11.2020 02:20


Mathematics, 18.11.2020 02:20

English, 18.11.2020 02:20

Mathematics, 18.11.2020 02:20

Mathematics, 18.11.2020 02:20

Spanish, 18.11.2020 02:20

Mathematics, 18.11.2020 02:20



Mathematics, 18.11.2020 02:20


History, 18.11.2020 02:20

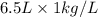 = 6.5 kg
= 6.5 kg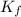 of water is 1.86 K kg/mol. And,
of water is 1.86 K kg/mol. And,