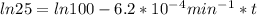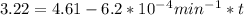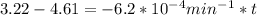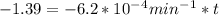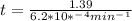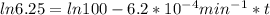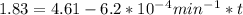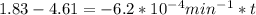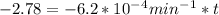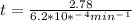Chemistry, 31.07.2019 20:30 YODIIZ6590
If the rate constant for the decomposition of n2o5 is 6.2 × 10−4/min, what is the half-life? (the rate law is first order in n2o5.) how long would it take for the concen- tration of n2o5 to decrease to 25% of its initial value? to 6.25% of its initial value?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 13:30
If a fast moving car making a loud noise approaches and moves past the person what will happen as the distance between the two increases?
Answers: 1
You know the right answer?
If the rate constant for the decomposition of n2o5 is 6.2 × 10−4/min, what is the half-life? (the r...
Questions


Social Studies, 13.12.2020 01:00

English, 13.12.2020 01:00

Mathematics, 13.12.2020 01:00




Mathematics, 13.12.2020 01:00



Spanish, 13.12.2020 01:00


Mathematics, 13.12.2020 01:00





English, 13.12.2020 01:00


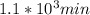 ,
, 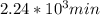 are taken for the concentration to decrease to 25% and
are taken for the concentration to decrease to 25% and 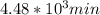 for the concentration to decrease to 6.25% .
for the concentration to decrease to 6.25% . 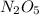 . For first order reaction, rate constant and half life are related to each other as:
. For first order reaction, rate constant and half life are related to each other as: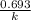
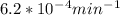
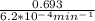
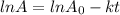
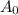 is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.
is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.