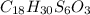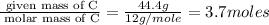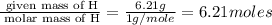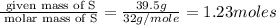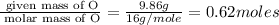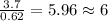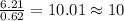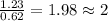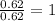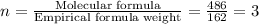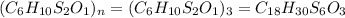Chemistry, 31.07.2019 20:30 live4dramaoy0yf9
6. allicin is the compound responsible for the characteristic smell of garlic. an analysis of the compound gives the following percent composition by mass: c: 44.4 percent; h: 6.21 percent; s: 39.5 percent; of: 9.86 percent. calculate its empirical formula. what is its molecular formula given that its molar mass is about 486g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
6. allicin is the compound responsible for the characteristic smell of garlic. an analysis of the co...
Questions

Mathematics, 19.05.2020 02:13








History, 19.05.2020 02:14





Biology, 19.05.2020 02:14



Mathematics, 19.05.2020 02:14

Spanish, 19.05.2020 02:14

Mathematics, 19.05.2020 02:14

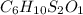 and the molecular of the compound is,
and the molecular of the compound is,