

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3

Chemistry, 23.06.2019 22:30
How much heat (in kj) is evolved in converting 1.00 mol of steam at 130.0 ∘c to ice at -55.0 ∘c? the heat capacity of steam is 2.01 j/g⋅∘c and of ice is 2.09 j/g⋅∘c?
Answers: 1
You know the right answer?
What is the ph of a solution prepared by mixing 25.00 ml of 0.10 m ch3co2h with 25.00 ml of 0.010 m...
Questions

Business, 21.11.2020 07:00

Social Studies, 21.11.2020 07:00


Mathematics, 21.11.2020 07:00

Mathematics, 21.11.2020 07:00

Mathematics, 21.11.2020 07:00

Mathematics, 21.11.2020 07:00


Mathematics, 21.11.2020 07:00

English, 21.11.2020 07:00



History, 21.11.2020 07:00

Biology, 21.11.2020 07:00


Mathematics, 21.11.2020 07:00


History, 21.11.2020 07:00

Mathematics, 21.11.2020 07:00

Chemistry, 21.11.2020 07:00

![pH = pKa + log\frac{[A-]}{[HA]} ----(1)](/tpl/images/0148/5540/36d6d.png)

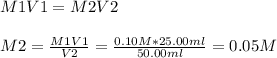
![pH = pKa + log\frac{[CH3COONa]}{[CH3COOH]} ----(1)](/tpl/images/0148/5540/1055b.png)
![pH = -logKa + log\frac{[CH3COONa]}{[CH3COOH]}](/tpl/images/0148/5540/5f73f.png)
![pH = 4.74 + log\frac{[0.005]}{[0.05]}](/tpl/images/0148/5540/63b4d.png)


