Chemistry, 30.07.2019 00:20 saabrrinnaaa
At 298 k, kc = 1.45 for the following reaction 2 brcl (g) br2(g) + cl2(g) a reaction mixture was prepared with the following initial concentrations. [brcl] = 0.0400 m, [br2] = 0.0300 m and [cl2] = 0.0300 m calculate their equilibrium concentrations.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
At 298 k, kc = 1.45 for the following reaction 2 brcl (g) br2(g) + cl2(g) a reaction mixture was pre...
Questions






Mathematics, 09.03.2021 01:00


Health, 09.03.2021 01:00

Mathematics, 09.03.2021 01:00



Mathematics, 09.03.2021 01:00


Biology, 09.03.2021 01:00

History, 09.03.2021 01:00

English, 09.03.2021 01:00




![1.5=K_{c}=\frac{[Br_{2}][Cl_{2}]}{[BrCl]^{2}}](/tpl/images/0148/5536/4f48c.png)
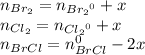
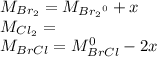
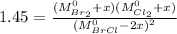
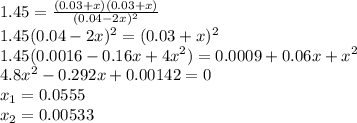
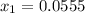 lacks of sense because it will give a negative concentration for BrCl, so the result is
lacks of sense because it will give a negative concentration for BrCl, so the result is 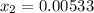 .
.