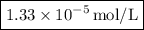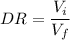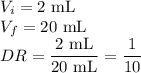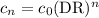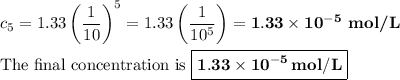Astudent performed a serial dilution on a stock solution of 1.33 m naoh. a 2.0 ml aliquot of the stock naoh (ms) was added to 18 ml of water to make the first dilution (m1). next, 2.0 ml of the m1 solution was added to 18 ml of water to make the second solution (m2). the same steps were repeated for a total of 5 times. what is the final concentration of naoh (m5)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

You know the right answer?
Astudent performed a serial dilution on a stock solution of 1.33 m naoh. a 2.0 ml aliquot of the sto...
Questions

Chemistry, 24.06.2019 07:20


Chemistry, 24.06.2019 07:20

Chemistry, 24.06.2019 07:20

Chemistry, 24.06.2019 07:20

Chemistry, 24.06.2019 07:20

Mathematics, 24.06.2019 07:20


English, 24.06.2019 07:20

Mathematics, 24.06.2019 07:20

Computers and Technology, 24.06.2019 07:20




Mathematics, 24.06.2019 07:20



Social Studies, 24.06.2019 07:20


Geography, 24.06.2019 07:20