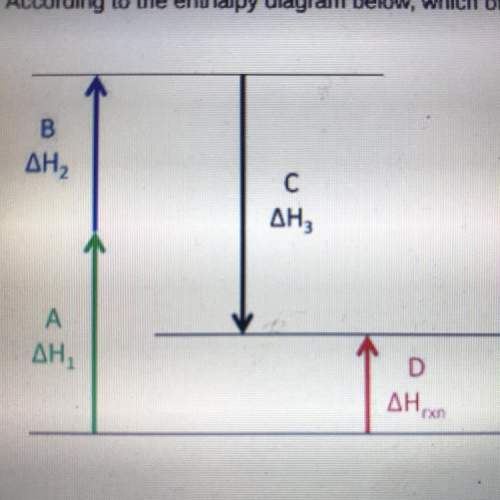
According to the enthalpy diagram below, which of the following statements is true.
a. arrows...

According to the enthalpy diagram below, which of the following statements is true.
a. arrows a and d represent the enthalpy of the intermediate chemical reactions.
b. arrow c represents the enthalpy of the overall chemical reaction.
c. arrow c indicates that the third intermediate reaction is exothermic.
d. arrow b indicates that the second intermediate reaction is exothermic.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Questions

Mathematics, 08.11.2019 16:31



Mathematics, 08.11.2019 16:31

Chemistry, 08.11.2019 16:31

English, 08.11.2019 16:31

History, 08.11.2019 16:31

History, 08.11.2019 16:31

Mathematics, 08.11.2019 16:31

Social Studies, 08.11.2019 16:31



Biology, 08.11.2019 16:31





Geography, 08.11.2019 16:31




