
Consider the following equation.
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s = 141.5 j/k. is this reaction spontaneous or nonspontaneous at high and low temperatures?
a. spontaneous at high temperatures, non-spontaneous at low temperatures
b. non-spontaneous at high and low temperatures
c. spontaneous at low temperatures, non-spontaneous at high temperatures
d. spontaneous at high and low temperatures

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

You know the right answer?
Consider the following equation.
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s...
fe2o3(s) + 3h2(g) → 2fe(s) + 3h2o(g)
h = 98.8 kj, and s...
Questions

Chemistry, 25.12.2019 13:31


Mathematics, 25.12.2019 13:31

History, 25.12.2019 13:31

Mathematics, 25.12.2019 13:31

Mathematics, 25.12.2019 13:31


English, 25.12.2019 13:31


Geography, 25.12.2019 13:31




Health, 25.12.2019 13:31




Biology, 25.12.2019 13:31

English, 25.12.2019 13:31

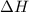 and the entropy change
and the entropy change 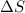 due to this reaction are positive. A chemical reaction will be spontaneous only if the change in its Gibbs Free Energy
due to this reaction are positive. A chemical reaction will be spontaneous only if the change in its Gibbs Free Energy 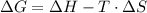 is negative.
is negative. 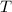 is the absolute temperature in degrees Kelvins.
is the absolute temperature in degrees Kelvins.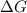 will initially be close to
will initially be close to 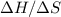 . The reaction will eventually become spontaneous.
. The reaction will eventually become spontaneous.

