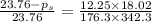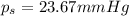The vapor pressure of water is 23.76 mm hg at 25 °c. a nonvolatile, nonelectrolyte that dissolves in water is sucrose. calculate the vapor pressure of the solution at 25 °c when 12.25 grams of sucrose, c12h22o11 (342.3 g/mol), are dissolved in 176.3 grams of water. water = h2o = 18.02 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1
You know the right answer?
The vapor pressure of water is 23.76 mm hg at 25 °c. a nonvolatile, nonelectrolyte that dissolves in...
Questions










Chemistry, 29.01.2020 05:41

Computers and Technology, 29.01.2020 05:41









Social Studies, 29.01.2020 05:41

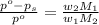
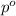 = vapor pressure of pure solvent (water) = 23.76 mmHg
= vapor pressure of pure solvent (water) = 23.76 mmHg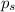 = vapor pressure of solution= ?
= vapor pressure of solution= ?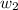 = mass of solute (sucrose) = 12.25 g
= mass of solute (sucrose) = 12.25 g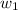 = mass of solvent (water) = 176.3 g
= mass of solvent (water) = 176.3 g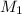 = molar mass of solvent (water) = 18.02 g/mole
= molar mass of solvent (water) = 18.02 g/mole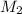 = molar mass of solute (sucrose) = 342.3 g/mole
= molar mass of solute (sucrose) = 342.3 g/mole