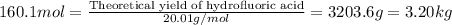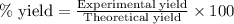Chemistry, 09.07.2019 00:50 robertschulte116
Hydrogen fluoride is used in the manufacture of freons (which destroy ozone in the stratosphere) and in the production of aluminum metal. it is prepared by the reaction caf2 + h2so4 → caso4 + 2hf in one process, 6.25 kg of caf2 is treated with an excess of h2so4 and yields 2.35 kg of hf. calculate the percent yield of hf. % yield

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

You know the right answer?
Hydrogen fluoride is used in the manufacture of freons (which destroy ozone in the stratosphere) and...
Questions

Mathematics, 21.03.2020 04:32









Mathematics, 21.03.2020 04:33





Social Studies, 21.03.2020 04:33



Biology, 21.03.2020 04:33

Mathematics, 21.03.2020 04:34

Mathematics, 21.03.2020 04:34

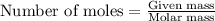 ....(1)
....(1) 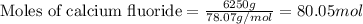
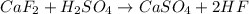
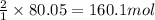 of hydrofluoric acid
of hydrofluoric acid