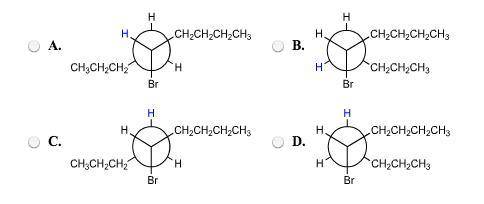
The elimination of 5−bromononane on treatment with potassium ethoxide is stereoselective. select the correct newman projections of 5−bromononane showing the conformations that lead to cis−4−nonene and trans−4−nonene, respectively. identify the proton that is lost in each case by selecting the newman projections that have this hydrogen colored blue. suggest a mechanistic explanation for the observed stereoselectivity. leads to cis−4−nonene:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
The elimination of 5−bromononane on treatment with potassium ethoxide is stereoselective. select the...
Questions

Social Studies, 01.07.2019 06:50

English, 01.07.2019 06:50



Chemistry, 01.07.2019 06:50

Biology, 01.07.2019 06:50

Business, 01.07.2019 06:50

History, 01.07.2019 06:50

Biology, 01.07.2019 06:50



Biology, 01.07.2019 06:50



Biology, 01.07.2019 06:50




Computers and Technology, 01.07.2019 06:50