
Chemistry, 08.07.2019 23:40 sairaanwar67
For the reaction h2(g) + co2(g) ⇌ h2o(g) + co(g) at 700ºc, kc = 0.534. calculate the number of moles of h2 that are present at equilibrium if a mixture of 0.300 mole of co and 0.300 mole of h2o is heated to 700ºc in a 10.0-l container.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

You know the right answer?
For the reaction h2(g) + co2(g) ⇌ h2o(g) + co(g) at 700ºc, kc = 0.534. calculate the number of moles...
Questions

History, 17.01.2021 04:40

Mathematics, 17.01.2021 04:40



Mathematics, 17.01.2021 04:40





Spanish, 17.01.2021 04:40

Mathematics, 17.01.2021 04:40




Mathematics, 17.01.2021 04:40

Mathematics, 17.01.2021 04:40



Mathematics, 17.01.2021 04:40

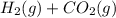 ⇄
⇄ 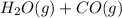 at 700ºC.
at 700ºC.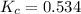
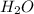 is heated to 700ºC.
is heated to 700ºC.![[H_2O]=\frac{300 mol}{10.0L}=30 M](/tpl/images/0067/3669/12641.png)
![H_2O=[H_2O]=\frac{300 mol}{10.0L}=30 M](/tpl/images/0067/3669/82ae5.png)
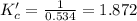
![K_c'=\frac{[H_2][CO_2]}{[CO][H_2O]}](/tpl/images/0067/3669/df550.png)
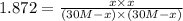
![[H_2]](/tpl/images/0067/3669/08a38.png)
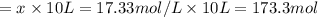
![[H_2]_{eq}=0.0173M](/tpl/images/0067/3669/87baf.png)
![Kc=\frac{[H_2O][CO]}{[H_2][CO_2]}](/tpl/images/0067/3669/4d854.png)
![[CO_2]_0=[H_2]_0=\frac{0.300mol}{10.0L} =0.030M](/tpl/images/0067/3669/89e63.png)
 due to the equilibrium, the law of mass action takes the following form:
due to the equilibrium, the law of mass action takes the following form: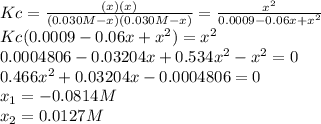
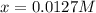
![[H_2]_{eq}=0.030M-0.0127M=0.0173M](/tpl/images/0067/3669/92f4c.png)



