
Be sure to answer all parts. nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: 2no(g) + o2(g) → 2no2(g)
in one experiment, 0.857 mol of no is mixed with 0.498 mol of o2.
determine which of the two reactants is the limiting reactant. calculate also the number of moles of no2 produced. limiting reactant: moles of no2 produced: moles

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
Be sure to answer all parts. nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2)...
Questions






Biology, 25.06.2019 18:40

History, 25.06.2019 18:40

English, 25.06.2019 18:40




Physics, 25.06.2019 18:40

Geography, 25.06.2019 18:40





Mathematics, 25.06.2019 18:50


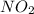 will be produced.
will be produced.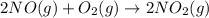
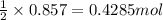 of
of 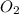
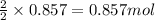 of
of 

