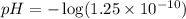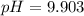Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
What is the ph of a solution with [h+] = 1.25 mc015-1.jpg 10–10 m?...
Questions

Mathematics, 31.03.2021 18:50



Chemistry, 31.03.2021 18:50

Mathematics, 31.03.2021 18:50


Mathematics, 31.03.2021 18:50


Mathematics, 31.03.2021 18:50

Mathematics, 31.03.2021 18:50

Mathematics, 31.03.2021 18:50




Mathematics, 31.03.2021 18:50

Mathematics, 31.03.2021 18:50

Mathematics, 31.03.2021 18:50


Mathematics, 31.03.2021 18:50

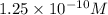
![pH=-\log [H^+]](/tpl/images/0195/0169/37e81.png)