Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
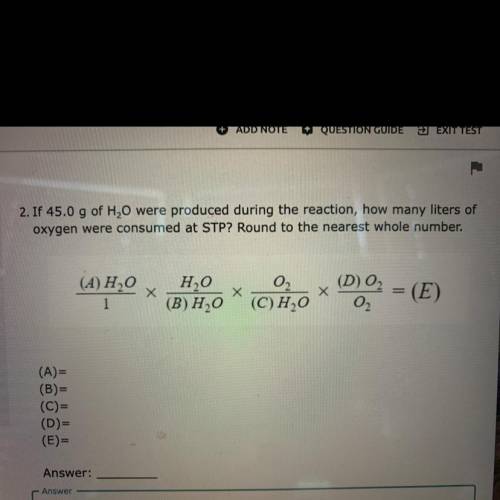
If 45.0 g of H2O were produced during the reaction, how many liters of
oxygen were consumed at STP?...
Questions

Physics, 02.09.2020 17:01

Geography, 02.09.2020 17:01

English, 02.09.2020 17:01

Mathematics, 02.09.2020 17:01



Biology, 02.09.2020 17:01




English, 02.09.2020 17:01





History, 02.09.2020 17:01


Computers and Technology, 02.09.2020 17:01

History, 02.09.2020 17:01

Mathematics, 02.09.2020 17:01