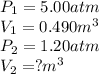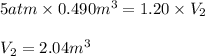Chemistry, 02.07.2019 03:10 ambriyaarmstrong01
Acontainer holds 0.490 m3 of oxygen at an absolute pressure of 5.00 atm. a valve is opened, allowing the gas to drive a piston, increasing the volume of the gas until the pressure drops to 1.20 atm. if the temperature remains constant, what new volume (in m3) does the gas occupy? hint m3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Acontainer holds 0.490 m3 of oxygen at an absolute pressure of 5.00 atm. a valve is opened, allowing...
Questions



English, 05.07.2019 17:10







Computers and Technology, 05.07.2019 17:10










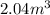
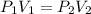 (at constant temperature)
(at constant temperature)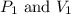 are initial pressure and volume.
are initial pressure and volume.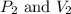 are final pressure and volume.
are final pressure and volume.