
Chemistry, 02.07.2019 03:10 wedderman6049
You have 3.00 l of a 3.00 m solution of nacl(aq) called solution a. you also have 2.00 l of a 2.00 m solution of agno3(aq) called solution b. you mix these solutions together, making solution c. hint: agcl is a precipitate. calculate the concentrations (in m) of the following ions in solution c. no3-

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:50
What type of reaction is illustrated? 2c12o5 = 2cl2 + 502
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
You have 3.00 l of a 3.00 m solution of nacl(aq) called solution a. you also have 2.00 l of a 2.00 m...
Questions


Mathematics, 10.11.2019 09:31





Biology, 10.11.2019 09:31

Chemistry, 10.11.2019 09:31





English, 10.11.2019 09:31

Mathematics, 10.11.2019 09:31


Mathematics, 10.11.2019 09:31

Physics, 10.11.2019 09:31



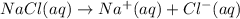
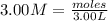
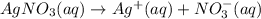
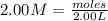
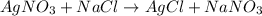
![[NO_{3}^-]=\frac{4 mol}{5 L}=0.8 mol/L](/tpl/images/0041/0466/43242.png)


