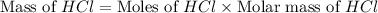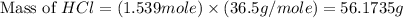Chemistry, 27.06.2019 19:30 helpmeplz11239
Determine the theoretical yield of hcl if 60.0 g of bc13 and 37.5 g of h20 are reacted according to the following balanced reaction. a possibly useful molar mass is bc13 117.16 g/mol. bc13(g)+3 h20(1) -- h3bo3(s)+3 hc1(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
Determine the theoretical yield of hcl if 60.0 g of bc13 and 37.5 g of h20 are reacted according to...
Questions

Mathematics, 25.05.2021 22:50


Chemistry, 25.05.2021 22:50

Mathematics, 25.05.2021 22:50

Mathematics, 25.05.2021 22:50

History, 25.05.2021 22:50


History, 25.05.2021 22:50


Mathematics, 25.05.2021 22:50

Mathematics, 25.05.2021 22:50


Biology, 25.05.2021 22:50



History, 25.05.2021 22:50

Spanish, 25.05.2021 22:50

Mathematics, 25.05.2021 22:50


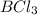 = 60 g
= 60 g
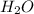 = 37.5 g
= 37.5 g
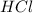 = 36.5 g/mole
= 36.5 g/mole
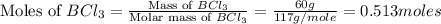
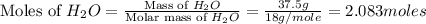
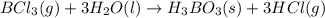
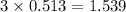 moles of
moles of