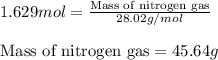Chemistry, 27.06.2019 19:30 38saferguson
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n204 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 92.02 g/mol, n2h4 32.05 g/mol n204) 2 n2h4(1)3 n2(g) + 4 h2o(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3


Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g...
Questions


Mathematics, 07.04.2021 20:50


Social Studies, 07.04.2021 20:50

Mathematics, 07.04.2021 20:50

Mathematics, 07.04.2021 20:50

Mathematics, 07.04.2021 20:50

Mathematics, 07.04.2021 20:50

Biology, 07.04.2021 20:50

Mathematics, 07.04.2021 20:50

Mathematics, 07.04.2021 20:50

Chemistry, 07.04.2021 20:50



Social Studies, 07.04.2021 20:50


Mathematics, 07.04.2021 20:50



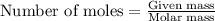 .....(1)
.....(1)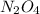
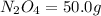
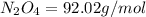
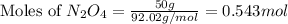
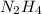
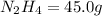
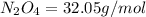
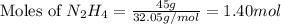
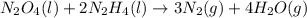
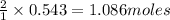 of
of 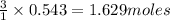 of nitrogen gas.
of nitrogen gas.