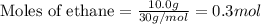Chemistry, 27.06.2019 10:10 Chatoloko231
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how many moles are present in this sample?
when answering the question, include the following:
state how to find the molar mass for the hydrocarbon.
state how you know if you need to multiply or divide by the molar mass.
give the correct number of significant figures and explain why the answer has that many significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how m...
Questions

Mathematics, 10.09.2019 04:30




Mathematics, 10.09.2019 04:30

Mathematics, 10.09.2019 04:30

Mathematics, 10.09.2019 04:30

Mathematics, 10.09.2019 04:30



Mathematics, 10.09.2019 04:30



Mathematics, 10.09.2019 04:30






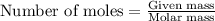
![[(2\times 12)+(6\times 1)]=30g/mol](/tpl/images/0022/9571/19171.png)