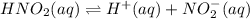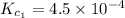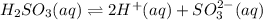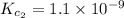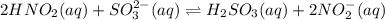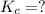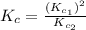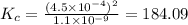Given the equilibrium constants for the following two reactions in aqueous solution at 25 ∘c hno2(aq)h2so3(aq)⇌⇌h+(aq) + no2−(aq)2h+(aq) + so32−(aq)kc = 4.5 × 10−4kc = 1.1 × 10−9 what is the value of kc for the reaction 2hno2(aq) + so32−(aq)⇌h2so3(aq) + 2no2−(aq)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2


Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
Given the equilibrium constants for the following two reactions in aqueous solution at 25 ∘c hno2(aq...
Questions




Mathematics, 11.06.2020 01:57




English, 11.06.2020 01:57



Mathematics, 11.06.2020 01:57




Mathematics, 11.06.2020 01:57


Mathematics, 11.06.2020 01:57



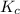 for the final reaction is, 184.09
for the final reaction is, 184.09