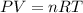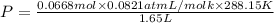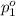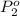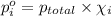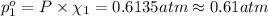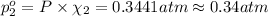Chemistry, 13.10.2019 01:10 sabrinamarie391
Agas mixture contains 1.20 g n2 and 0.77 g o2 in a 1.65-l container at 15 ∘c. part a calculate the mole fraction of n2. express your answer using two significant figures. x1 x 1 = nothing request answer part b calculate the mole fraction of o2. express your answer using two significant figures. x2 x 2 = nothing request answer part c calculate the partial pressure of n2. express your answer using two significant figures. p1 p 1 = nothing atm request answer part d calculate the partial pressure of o2. express your answer using two significant figures. p2 p 2 = nothing atm request answer provide feedback

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Agas mixture contains 1.20 g n2 and 0.77 g o2 in a 1.65-l container at 15 ∘c. part a calculate the m...
Questions










Mathematics, 29.02.2020 02:51







Mathematics, 29.02.2020 02:51



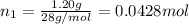
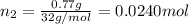
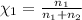
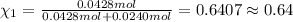
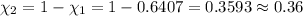
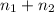 = 0.0428 mol + 0.0240 mol = 0.0668 mol
= 0.0428 mol + 0.0240 mol = 0.0668 mol