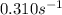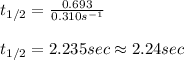Chemistry, 19.11.2019 10:31 PlaneGamer5678
The initial concentration of a in the first-order reaction 4a→4b+c is 0.933 mol l−1. given that the rate constant is 0.310 s−1, what is the half-life of the reaction in seconds? remember to use correct significant figures in your answer (round your answer to the nearest hundredth). do not include units in your response.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
The initial concentration of a in the first-order reaction 4a→4b+c is 0.933 mol l−1. given that the...
Questions

Biology, 15.10.2019 22:00


Mathematics, 15.10.2019 22:00




English, 15.10.2019 22:00

Mathematics, 15.10.2019 22:00

Mathematics, 15.10.2019 22:00



Health, 15.10.2019 22:00

Mathematics, 15.10.2019 22:00

Social Studies, 15.10.2019 22:00

Social Studies, 15.10.2019 22:00


Mathematics, 15.10.2019 22:00

Biology, 15.10.2019 22:00


Mathematics, 15.10.2019 22:00

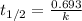
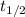 = half-life of the reaction
= half-life of the reaction