
Chemistry, 25.01.2020 00:31 brittanysanders
How much heat is released when 105g of steam at 100.0c is cooled to ice at -15c? enthalpy of vaporization of water is 40.67kj/mol, the enthalpy of fusion of water is 6.01kj/mol, the molar heat capacity of water is 75.4j/(mol. c) and the molar heat capacity of ice is 36.4j/(mol. c)

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3
You know the right answer?
How much heat is released when 105g of steam at 100.0c is cooled to ice at -15c? enthalpy of vapori...
Questions







Chemistry, 25.12.2020 23:40





Social Studies, 25.12.2020 23:40



Computers and Technology, 25.12.2020 23:40


Computers and Technology, 25.12.2020 23:50



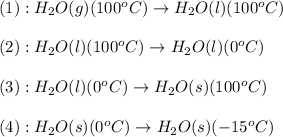
![\Delta H=n\times \Delta H_{condensation}+[n\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{freezing}+[n\times c_{p,s}\times (T_{final}-T_{initial})]](/tpl/images/0469/7239/e91c9.png)
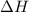 = enthalpy change = ?
= enthalpy change = ?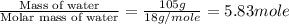
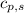 = specific heat of solid water =
= specific heat of solid water = 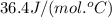
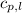 = specific heat of liquid water =
= specific heat of liquid water = 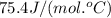
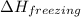 = enthalpy change for freezing = enthalpy change for fusion = - 6.01 KJ/mole = - 6010 J/mole
= enthalpy change for freezing = enthalpy change for fusion = - 6.01 KJ/mole = - 6010 J/mole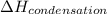 = enthalpy change for condensation = enthalpy change for vaporization = -40.67 KJ/mole = -40670 J/mole
= enthalpy change for condensation = enthalpy change for vaporization = -40.67 KJ/mole = -40670 J/mole![\Delta H=5.83mole\times -40670J/mole+[5.83mole\times 75.4J/(mol.^oC)\times (0-100)^oC]+5.83mole\times -6010J/mole+[5.83mole\times 36.4J/(mol.^oC)\times (-15-0)^oC]](/tpl/images/0469/7239/b7d7d.png)
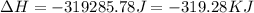 (1 KJ = 1000 J)
(1 KJ = 1000 J)

