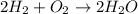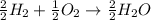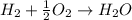Chemistry, 29.06.2019 11:20 dakotaadkins1818
Will mark ! read the chemical equation. 2h2 + o2 → 2h2o which of the following statements would be correct if one mole of hydrogen was used in this reaction? a. one mole of oxygen was used in this reaction. b. two moles of oxygen were used in this reaction. c. one mole of water was produced from this reaction. d. two moles of water were produced from this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Will mark ! read the chemical equation. 2h2 + o2 → 2h2o which of the following statements would be...
Questions




Mathematics, 05.05.2020 18:26

Health, 05.05.2020 18:26


History, 05.05.2020 18:26

English, 05.05.2020 18:26


Engineering, 05.05.2020 18:26


Social Studies, 05.05.2020 18:26




History, 05.05.2020 18:27