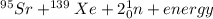Chemistry, 29.06.2019 14:10 phsycotic121
[oss.03]the models below represent nuclear reactions. the atoms on the left of the equal sign are present before the reaction and the atoms on the right of the equal sign are produced after the reaction. model 1: atom 1 + atom 2 = atom 3 + energymodel 2: atom 4 = atom 5 + atom 6 + energywhich of these statements is most likely correct about the two models? both models show reactions which use up energy in the sun. both models show reactions which produce energy in the sun. model 1 shows reactions in the sun and model 2 shows reactions in the nuclear power plants. model 1 shows reactions in the nuclear power plants and model 2 shows reactions in the sun.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
[oss.03]the models below represent nuclear reactions. the atoms on the left of the equal sign are pr...
Questions

Mathematics, 25.07.2019 09:00

Chemistry, 25.07.2019 09:00

Business, 25.07.2019 09:00


Social Studies, 25.07.2019 09:00



Mathematics, 25.07.2019 09:00












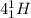 →
→ 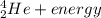 + some other particles
+ some other particles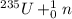 →
→