Chemistry, 03.02.2020 01:55 farhansayeed11
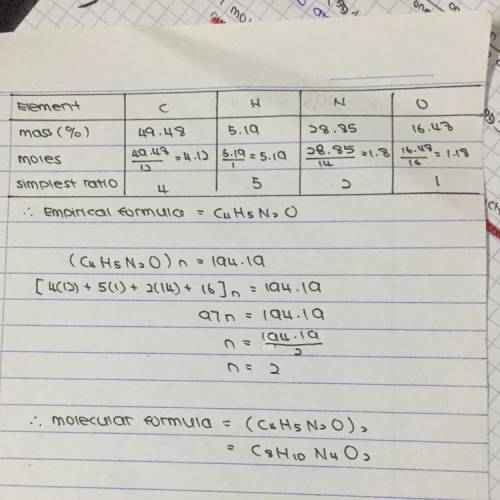
Caffeine has the following percent composition: carbon 49.48%, hydrogen 5.19%, oxygen 16.48% and nitrogen 28.85%. its molecular weight is 194.19 g/mol. what is its molecular formula?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
Caffeine has the following percent composition: carbon 49.48%, hydrogen 5.19%, oxygen 16.48% and ni...
Questions

Mathematics, 15.02.2021 20:20





Mathematics, 15.02.2021 20:20



Mathematics, 15.02.2021 20:20


Physics, 15.02.2021 20:20






Mathematics, 15.02.2021 20:20

Mathematics, 15.02.2021 20:20

English, 15.02.2021 20:20