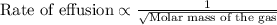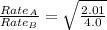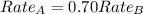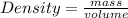Chemistry, 29.09.2019 01:30 zmoore3793
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. molar mass comparison gas molar mass a 4.00 g/mol b 2.01 g/mol which statement describes the density and effusion of both gases at stp? gas a has a higher density and effuses faster than gas b. gas a has a higher density and effuses slower than gas b. gas a has a lower density and effuses faster than gas b. gas a has a lower density and effuses slower than gas b.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. mola...
Questions



Mathematics, 16.09.2021 02:10

Mathematics, 16.09.2021 02:10

Mathematics, 16.09.2021 02:10


Mathematics, 16.09.2021 02:10


Physics, 16.09.2021 02:10

Physics, 16.09.2021 02:10


Mathematics, 16.09.2021 02:10

History, 16.09.2021 02:10


Social Studies, 16.09.2021 02:10

Mathematics, 16.09.2021 02:10

English, 16.09.2021 02:10


Mathematics, 16.09.2021 02:10