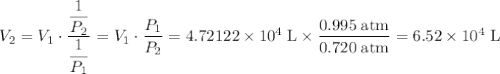Chemistry, 13.10.2019 15:50 AphEngland
Aweather balloon is filled with helium that occupies a volume of 5.57 104 l at 0.995 atm and 32.0°c. after it is released, it rises to a location where the pressure is 0.720 atm and the temperature is -14.5°c. what is the volume of the balloon at that new location?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2


Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
Aweather balloon is filled with helium that occupies a volume of 5.57 104 l at 0.995 atm and 32.0°c....
Questions



Mathematics, 05.05.2020 01:19

Mathematics, 05.05.2020 01:19


Mathematics, 05.05.2020 01:19


Mathematics, 05.05.2020 01:19

Mathematics, 05.05.2020 01:19


Biology, 05.05.2020 01:19


Business, 05.05.2020 01:19





Mathematics, 05.05.2020 01:19


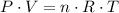 ,
,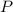 is the pressure of the gas,
is the pressure of the gas,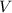 is the volume of the gas,
is the volume of the gas, 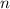 is the number of gas particles in the gas,
is the number of gas particles in the gas, 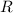 is the ideal gas constant, and
is the ideal gas constant, and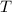 is the absolute temperature of the gas in degrees Kelvins.
is the absolute temperature of the gas in degrees Kelvins.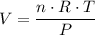 .
. 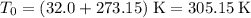 ,
,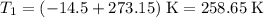 .
.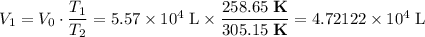 .
.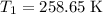 and change the pressure on the gas:
and change the pressure on the gas: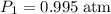 ,
,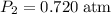 .
.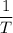 if both
if both