
Chemistry, 25.06.2019 22:30 tatibean26
Consider the chemical equations shown here.2no2(g) > 2no(g) + o2(g)2no(g) > n2(g) + o2(g)n2(g) + 2o(g) > n2o4(g)what is the equation for the overall reaction obtained by adding these equations? 2no2(g) > n2o4(g)2n204(g) + 2no(g) > 2no2(g) + 02(g)n2 + o2(g) + 2no(g) > n2o4 (g)the answer is a

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1


Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 23.06.2019 14:20
Neutral atoms of argon, atomic number 18, have the same number of electrons as each of the following items except: cl- s -2 k+ ca +2 ne
Answers: 2
You know the right answer?
Consider the chemical equations shown here.2no2(g) > 2no(g) + o2(g)2no(g) > n2(g) + o2(g)n2(...
Questions


Mathematics, 20.07.2021 09:20

Mathematics, 20.07.2021 09:20

Physics, 20.07.2021 09:20

Mathematics, 20.07.2021 09:20

Social Studies, 20.07.2021 09:20

Chemistry, 20.07.2021 09:20




Computers and Technology, 20.07.2021 09:20

English, 20.07.2021 09:20


History, 20.07.2021 09:20




Mathematics, 20.07.2021 09:20


Mathematics, 20.07.2021 09:20


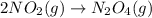
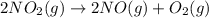 ...(1)
...(1)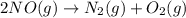 ...(2)
...(2)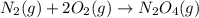 ...(3)
...(3)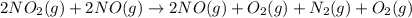
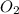 is on same side it will get added up.
is on same side it will get added up.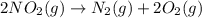 ..(4)
..(4)

