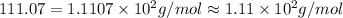Chemistry, 26.06.2019 06:30 laylalucifer
What is the molar mass of cacl2? 37.0 g/mole 54.0 g/mole 75.0 g/mole 1.11 x 102 g/mole

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
What is the molar mass of cacl2? 37.0 g/mole 54.0 g/mole 75.0 g/mole 1.11 x 102 g/mole...
Questions

Social Studies, 06.11.2020 07:30


English, 06.11.2020 07:30

History, 06.11.2020 07:30

English, 06.11.2020 07:30


Mathematics, 06.11.2020 07:30

History, 06.11.2020 07:30

Chemistry, 06.11.2020 07:30

Physics, 06.11.2020 07:30

Mathematics, 06.11.2020 07:30


Mathematics, 06.11.2020 07:30




Mathematics, 06.11.2020 07:30

Mathematics, 06.11.2020 07:30


Mathematics, 06.11.2020 07:30

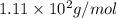
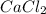 :
: