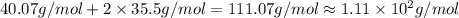Chemistry, 26.06.2019 06:30 ItzJuztWillie
What is the molar mass of cacl2? 37.0 g/mole 54.0 g/mole 75.0 g/mole 1.11 x 102 g/mole

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 09:00
Astream of surface water reaches a porous portion of sediment and seeps into the ground. this water eventually joins a large reservoir of water located beneath the earth's surface. the example above describes the interacting with the a. cryosphere; biosphere b. hydrosphere; biosphere c. hydrosphere; geosphere d. cryosphere; geosphere
Answers: 3
You know the right answer?
What is the molar mass of cacl2? 37.0 g/mole 54.0 g/mole 75.0 g/mole 1.11 x 102 g/mole...
Questions

History, 24.11.2020 05:50


English, 24.11.2020 05:50

Mathematics, 24.11.2020 05:50


History, 24.11.2020 05:50


English, 24.11.2020 05:50


World Languages, 24.11.2020 05:50


Biology, 24.11.2020 05:50


Mathematics, 24.11.2020 05:50

History, 24.11.2020 05:50



Mathematics, 24.11.2020 05:50

English, 24.11.2020 05:50

Mathematics, 24.11.2020 05:50

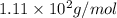
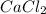 there are two chlorine atoms and one calcium atom.
there are two chlorine atoms and one calcium atom.