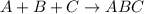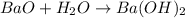Chemistry, 26.06.2019 10:30 LeahAshe123
What is the number of moles in 500 l of he gas at stp? 0.05 0.2 22 90 what is the percent of the composition of chromium bacro4? 4.87% 9.47% 20.5% 25.2% what is the empirical formula of a substance that is 53.5% c, 15.5% h, 31.1% n by weight c3hn2 c4h14n2 c2h7n ch4n7 the product of a combination reaction is ba(oh)2. if one of the reactants is h2o, what is the other reactant? ba2o bao bah bao2 which of the following is true about single replacement reactions they are restricted to metals they involve a single product two reactants produce two products any metal replaces any other metal. once all answered and finished i will post the whole exam. only if they are answered within 20 minutes. 11: 25am right now.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2
You know the right answer?
What is the number of moles in 500 l of he gas at stp? 0.05 0.2 22 90 what is the percent of the co...
Questions

Biology, 17.09.2019 15:00


English, 17.09.2019 15:00

Chemistry, 17.09.2019 15:00


Physics, 17.09.2019 15:00

Mathematics, 17.09.2019 15:00


History, 17.09.2019 15:00

Mathematics, 17.09.2019 15:00



Mathematics, 17.09.2019 15:00

Computers and Technology, 17.09.2019 15:00

Computers and Technology, 17.09.2019 15:00



Mathematics, 17.09.2019 15:00

Biology, 17.09.2019 15:00

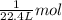
![]\frac{1}{22.4 L}\times 500L =22.32 mol\approx 22 mol](/tpl/images/0019/1602/e65bd.png)
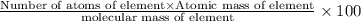
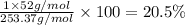
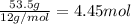
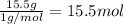
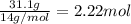
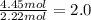
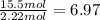
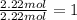
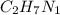 .
.